
CURE Childhood Cancer is thrilled to announce a remarkable achievement in our 50-year mission to end childhood cancer: a $5.6 million investment in lifesaving research studies.
These 13 innovative research projects are led by renowned scientists at premier pediatric cancer research institutions across the country and target the most urgent challenges facing young patients. What makes this investment so powerful is that every project is designed with the goal of helping children within the next 2-3 years – not decades from now. All proposals underwent rigorous review by CURE’s Peer Review Committee, comprised of practicing pediatric oncologists and academic researchers.
For families facing devastating diagnoses, this funding represents real hope, not someday, but soon. Thank you for making this breakthrough investment possible.
“We are proud to partner with some of the brightest minds in pediatric cancer research – scientists who have devoted their lives to solving the most difficult challenges children with cancer face,” said Kristin Connor, CEO of CURE Childhood Cancer. “With so little federal funding directed toward pediatric cancers, our support is often the catalyst that allows critical science to move forward. We are laser-focused on changing the odds for children with few treatment options and on discovering therapies that don’t leave devastating lifelong side effects. It’s because of our incredible community of supporters that we can fuel this progress and continue pushing toward the day when every child has the chance to be cured.”
Thank you for being an essential part of this journey.
CURE’s 2025 RESEARCH AWARDS
Early Investigator Awards
Emily Heikamp, MD, PhD, Dana-Farber Cancer Institute
Targeting chromatin regulators of oncogenic transcription in NUP98-rearranged leukemia
Nathaniel Mabe, PhD, Purdue University
Selective targeting of epigenetic pathways underlying drug tolerant persistence in neuroblastoma
Palaniraja Thandapani, PhD, The University of Texas MD Anderson Cancer Center
Targeting Proline tRNA Biogenesis as a Therapeutic Strategy in NOTCH1-Driven T-ALL
Translation to CURE Awards
Manoj Bhasin, PhD, MS, Emory University
Interrogation of mast cells as a high-risk biomarker in core binding factor mutated pediatric acute myeloid leukemia
Kelly Goldsmith, MD, Emory University
Companion Molecular Imaging for PTK7 Targeted Immunotherapies in Pediatric Solid Tumors
Rintaro Hashizume, MD, PhD, University of Alabama at Birmingham
Intranasal Delivery of Targeted Nanotherapeutics and Oncolytic Virus in Pediatric Glioma
Raushan Kurmasheva, PhD, University of Texas Health Science Center at San Antonio
Advancing Innovative and Effective Therapies for Children with Malignant Rhabdoid Tumors
Kathy Fange Liu, PhD, University of Pennsylvania
METTL3-targeting ASOs and synthetic lethality approaches in pediatric neuroblastoma
Paul Sondel, MD, PhD, University of Wisconsin-Madison
Novel GD2/B7-H3 Bispecific Antibody with Agonist CD40 Antibody, Epigenetic Modifier Inhibitors and Checkpoint Blockade to Improve Treatment Efficacy for High-Risk Neuroblastoma
Michael Verneris, MD, University of Colorado Denver
Translational Strategies To Enhance B7-H3-CXCR2 CAR T Homing and Function in Sarcoma
Elvin Wagenblast, PhD, Icahn School of Medicine at Mount Sinai
PR Domain Inhibition to Target Leukemia Stem Cells in Pediatric Acute Myeloid Leukemia
Muxiang Zhou, MD, Emory University
Dual inhibition of MDM2 and tubulin for precision treatment of acute myeloid leukemia
Precision Medicine Program, Children’s Healthcare of Atlanta
A program leveraging genomic sequencing for pediatric patients with high-risk tumors, with the goal of identifying alterations that can impact therapies and improve outcomes.

 Genetic sequencing through CURE’s Precision Medicine Program revealed crucial information: her tumor carried a gene fusion called KIAA1549: BRAF. This discovery proved to be both a challenge and an opportunity. While traditional treatments would likely be less effective because of this mutation, the discovery opened the door to targeted therapies called MEK inhibitors that could block the activity of proteins that cause tumor growth.
Genetic sequencing through CURE’s Precision Medicine Program revealed crucial information: her tumor carried a gene fusion called KIAA1549: BRAF. This discovery proved to be both a challenge and an opportunity. While traditional treatments would likely be less effective because of this mutation, the discovery opened the door to targeted therapies called MEK inhibitors that could block the activity of proteins that cause tumor growth.


 Ally received her first dose of chemotherapy within 16 hours of diagnosis. Over the two years of treatment that followed, Ally struggled with almost every possible side effect. She suffered three life-threatening infections, temporarily lost the ability to walk, and struggled to rebuild her immune system after every round of chemo.
Ally received her first dose of chemotherapy within 16 hours of diagnosis. Over the two years of treatment that followed, Ally struggled with almost every possible side effect. She suffered three life-threatening infections, temporarily lost the ability to walk, and struggled to rebuild her immune system after every round of chemo.


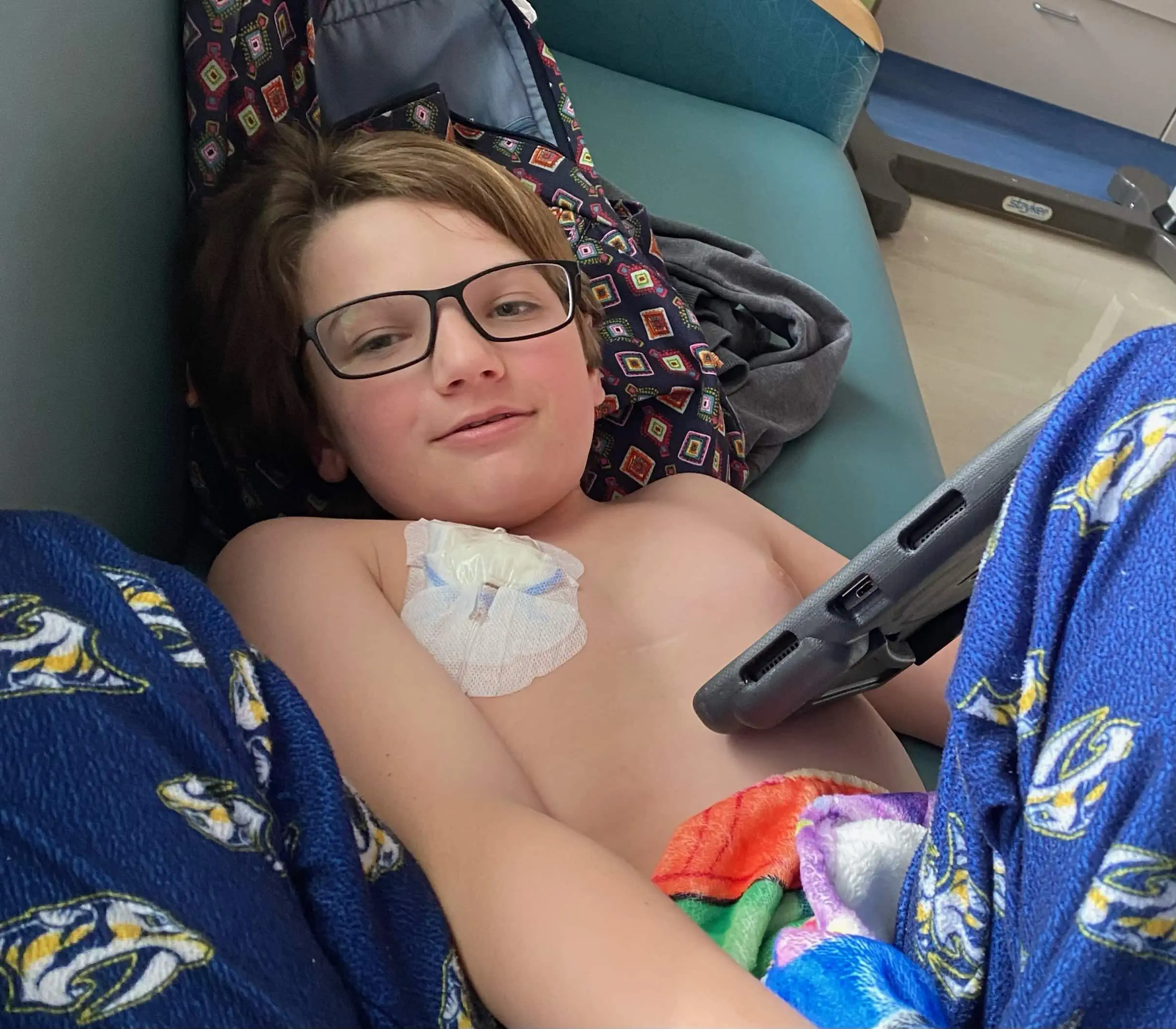 Easton had surgery to remove as much of the tumor as possible, followed by eight weeks of proton radiation in Jacksonville, Florida. His family was thrilled when a follow-up scan showed no presence of the tumor.
Easton had surgery to remove as much of the tumor as possible, followed by eight weeks of proton radiation in Jacksonville, Florida. His family was thrilled when a follow-up scan showed no presence of the tumor.

