
Easton has been fighting cancer for most of his twelve years. When he was 21 months old, he was constantly sick. His pediatrician noticed that his sickness was abnormal – he vomited more frequently in the mornings and while sitting in his car seat. A scan revealed a large tumor on Easton’s brain stem near the area that controls swallowing and nausea.
“The fact that Easton would get sick after being in his car seat made our pediatrician suspicious,” recalled his mother, Jill. “If the car seat hadn’t put pressure on the tumor, it might have taken longer to find.”
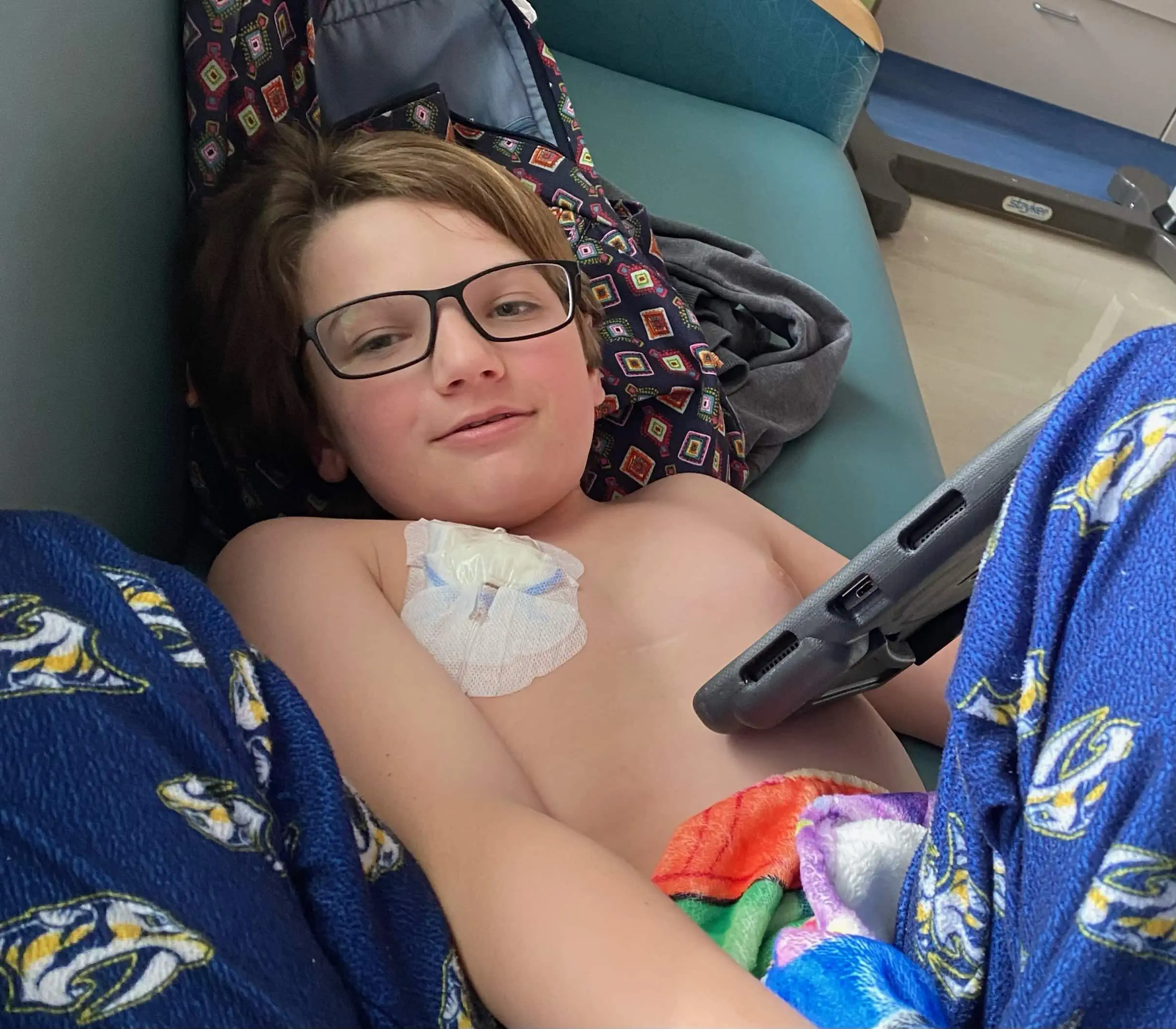
“We thought it was over,” said Jill. “We were told that if we had five years of clear scans, we wouldn’t have to worry about it again. Easton had four years of clear scans. But the cancer came back during the fifth year.”
Easton started treatment all over again in 2017 and experienced horrible side effects from the chemotherapy and radiation. Because the tumor grows around the area of the brain that controls swallowing, he has always had challenges eating. The awful mouth sores from his chemo caused him to be on a mostly liquid diet, and he had trouble maintaining weight.
In May 2022, Easton underwent a very risky surgery to try and remove the last part of the tumor. The surgery was unsuccessful. As he started recovering, Easton fell out of bed in the middle of the night. His parents rushed him to the emergency room, where doctors found that Easton’s brain was having a reaction to the glue that was used on his skull after surgery. He would need another risky brain surgery.
“This time, I felt like it was getting away from us and it was the beginning of the end,” said Jill. “He had been on some form of treatment for five years, and the tumor always found its way around it.”
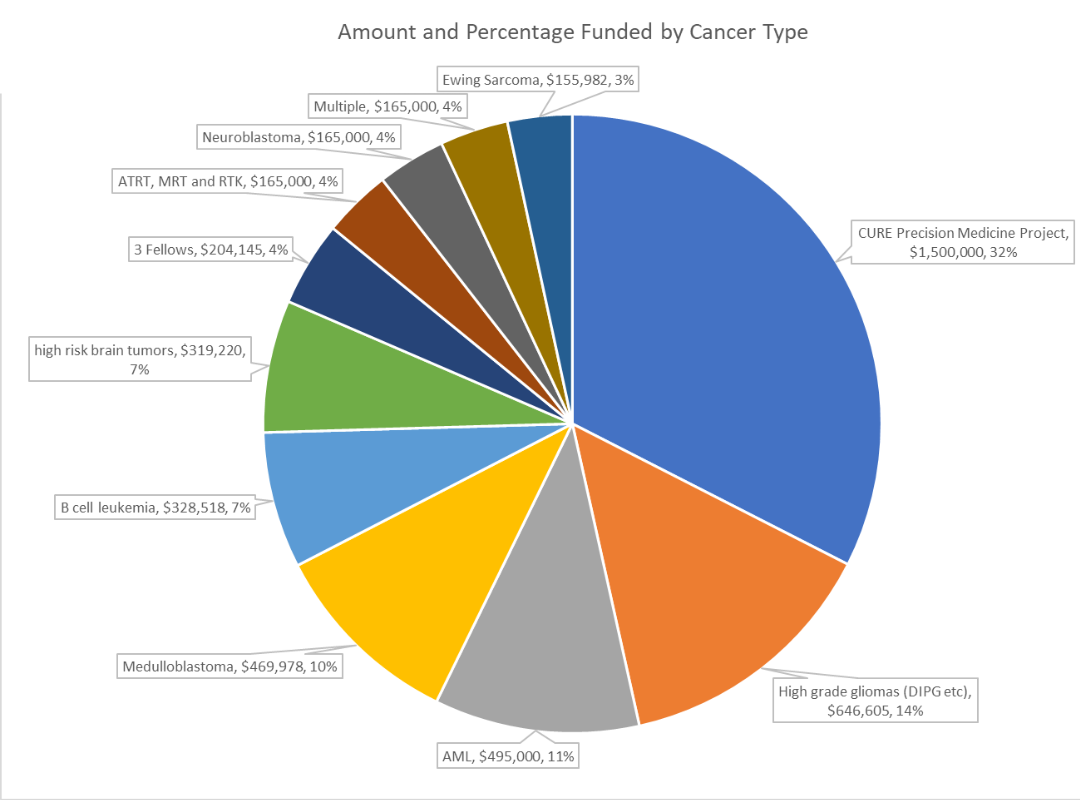
But thanks to funding from CURE Childhood Cancer, Easton’s doctors have a new tool in their toolbox. CURE’s funds would pay for Easton’s tumor to be genetically mapped to see if his cancer involved any genetic mutations that could be targeted.
The genetic mapping revealed that a protein was feeding Easton’s tumor, causing it to grow. Doctors found an open clinical trial using a chemotherapy to inhibit this specific protein to prevent further tumor growth. Easton was immediately enrolled in the trial, and the results have been astounding. After four months, a scan showed that the inside of Easton’s tumor appeared to be dying. Three months later, the tumor is much smaller and is collapsing in on itself.
“The best news is that the tumor is dying. But also, he has no side effects. He can do the things a twelve-year-old should do while on this treatment,” shared Jill. “We’re still early in the process, but it has saved his life – at least at this point. I finally have hope for the first time in years. We are so encouraged and thankful to his doctors and to CURE for investing in precision medicine.”
Take the next step to support research that will help save kids with cancer… kids like Easton.







 Dr. Jensen started his career in the late 1990s at City of Hope National Medical Center in Los Angeles, where his research looked at the technology to take immune cells from a cancer patient and genetically modify them to recognize and attack cancer cells. This is something the patient’s natural immune system can’t do. His research was groundbreaking at that time, and his lab had to build everything from scratch.
Dr. Jensen started his career in the late 1990s at City of Hope National Medical Center in Los Angeles, where his research looked at the technology to take immune cells from a cancer patient and genetically modify them to recognize and attack cancer cells. This is something the patient’s natural immune system can’t do. His research was groundbreaking at that time, and his lab had to build everything from scratch.



 Marc and his wife, Andrea, would soon learn that DIPG is a rare brain cancer for which there is no known cure. As if that wasn’t bad enough, Raegan had the worst possible genetic mutation and was given a prognosis of 6-9 months. Both Marc and Andrea work in the medical field and began scouring the internet for information and potential clinical trials.
Marc and his wife, Andrea, would soon learn that DIPG is a rare brain cancer for which there is no known cure. As if that wasn’t bad enough, Raegan had the worst possible genetic mutation and was given a prognosis of 6-9 months. Both Marc and Andrea work in the medical field and began scouring the internet for information and potential clinical trials.

 Just after her third birthday, Madeline had terrible leg pain and refused to walk, so Bethany took her back to the hospital. Madeline’s blood work showed that inflammation markers were very high, so doctors ordered a total body scan. They found a football-sized mass in her abdomen that had smashed her bladder and pushed into her intestines, kidney, and liver. A biopsy of the mass confirmed a diagnosis of neuroblastoma.
Just after her third birthday, Madeline had terrible leg pain and refused to walk, so Bethany took her back to the hospital. Madeline’s blood work showed that inflammation markers were very high, so doctors ordered a total body scan. They found a football-sized mass in her abdomen that had smashed her bladder and pushed into her intestines, kidney, and liver. A biopsy of the mass confirmed a diagnosis of neuroblastoma. Another part of the APMP is the genetic predisposition program, which provides care for children who are at risk for developing cancer due to a cancer predisposition syndrome or a family history of cancer. Madeline’s younger sister, Sedona, has a known genetic disorder called hemihypertrophy. So she was referred to the genetic predisposition clinic to see if her genetic mutation was the same as Madeline’s. If a genetic link between the two was revealed, it might indicate that Sedona had a high risk of developing cancer in the future.
Another part of the APMP is the genetic predisposition program, which provides care for children who are at risk for developing cancer due to a cancer predisposition syndrome or a family history of cancer. Madeline’s younger sister, Sedona, has a known genetic disorder called hemihypertrophy. So she was referred to the genetic predisposition clinic to see if her genetic mutation was the same as Madeline’s. If a genetic link between the two was revealed, it might indicate that Sedona had a high risk of developing cancer in the future.


